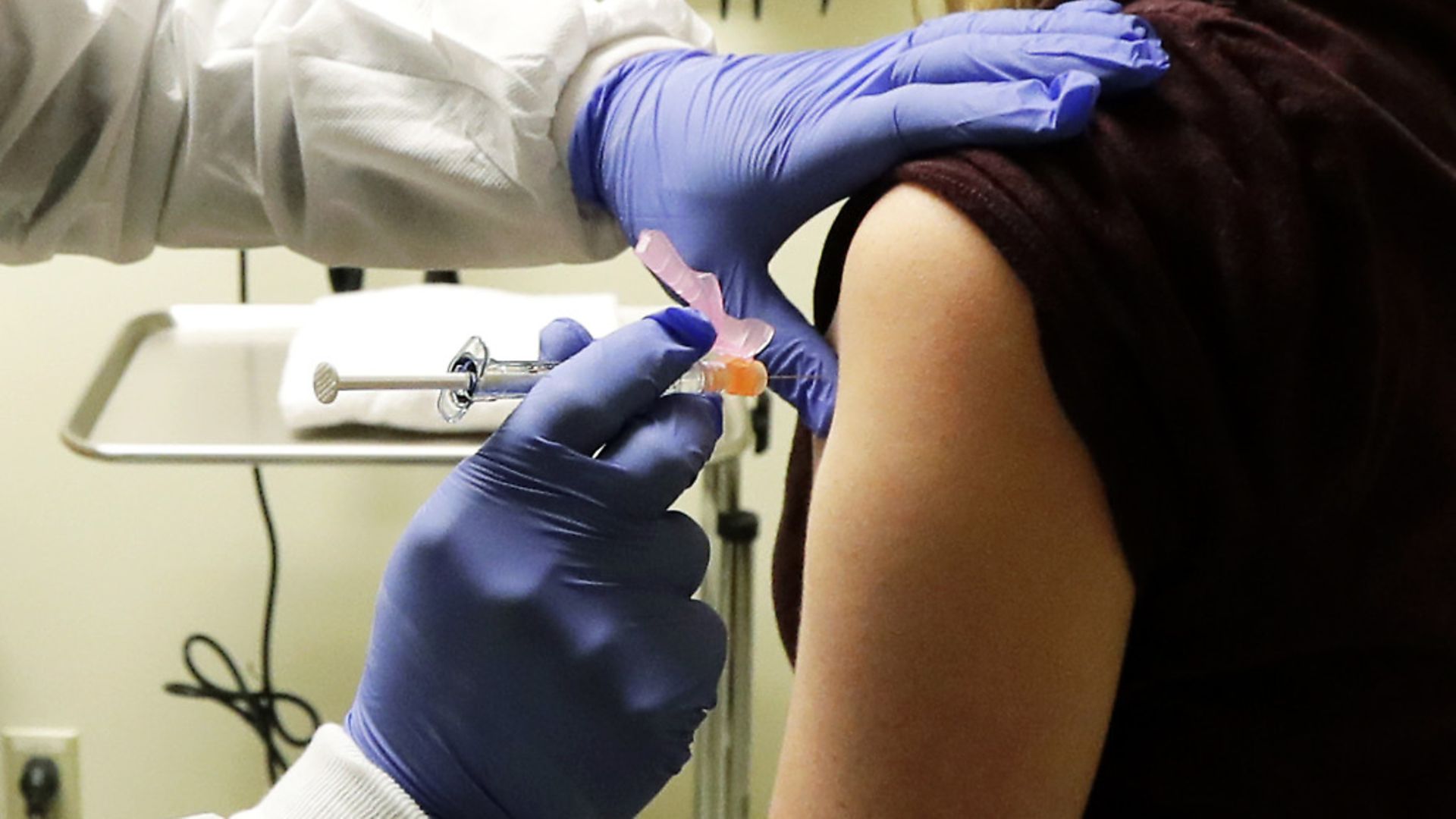
UK researchers look set to test a coronavirus vaccine on humans as early as next month as part of global effort to find treatment for the pandemic.
Safety trials are due to begin in April as a drug developed by Oxford University is anticipated to be given the green light to move to human testing well ahead of the usual time frame for drug development.
Researchers expect the emergency work will be accelerated as similar vaccines developed for more than 10 different diseases have been proved safe in human trials with thousands of subjects aged from one week to 90 years old.
A team led by professor Sarah Gilbert at the university’s Jenner Institute started designing what is expected to be Britain’s first Covid-19 vaccine candidate on January 10.
Animal tests of the same drug are only due to take place at Public Health England’s (PHE) specialised facilities at Porton Down in Wiltshire from next week. Normally all animal tests have to be completed before human trials can begin.
Jenner Institute director Professor Adrian Hill told the Guardian: ‘We are conscious that a vaccine is needed as soon as possible and certainly by June-July, when we expect a big peak in mortality.
‘This is not a normal situation. We will follow all standard trial safety requirements, but as soon as we have a vaccine that’s working, we anticipate there will be an accelerated pathway to get it deployed to save lives.
‘The more vaccine we can provide sooner, the better.’
Although the first clinical trials have not even taken place, vaccine production is already ‘being scaled up ready for larger trials, and potentially, future deployment’, the Jenner Institute said.
Meanwhile, PHE scientists and teams at Liverpool and Bristol universities have created an exact synthetic replica of Covid-19 which will allow the health agency to ‘carry out rigorous evaluation and testing of vaccines and treatments that enter the clinic’, PHE said.
PHE said its facilities allow it to obtain a ‘rigorous understanding of vaccine and therapeutic safety and efficacy, before new interventions enter human trials.’
Countries and pharmaceutical firms around the world are locked in a race to develop a vaccine to arrest the spread of the new pandemic.
Clinical trials are already under way in Europe, America and China, with 1,000 scientists in China backed by the country’s military science unit working on a treatment, the New York Times reported.
But specialists have warned of potential dangers in rushing to find vaccines.
Professor Stephen Turner, from Monash University’s department of Microbiology, in Australia, said risks include producing a vaccine which does not work for the whole population or has undetected side-effects.
Have your say
Send your letters for publication to The New European by emailing letters@theneweuropean.co.uk and pick up an edition each Thursday for more comment and analysis. Find your nearest stockist here or subscribe to a print or digital edition for just £13. You can also join our readers' Facebook group to keep the discussion and debate going with thousands of fellow pro-Europeans.
But he added: ‘If the public need is great enough, then I think bringing drugs forward in the approval process is a viable option.
‘As with a vaccine, or anything you put into people, you would need to show safety and efficacy before allowing it as a therapeutic.’
oris Johnson said the government’s plans to purchase antibody tests on a massive scale to detect if someone has had the disease were ‘a game changer’.
Johnson said ‘hundreds of thousands’ of kits could be bought if the tests prove effective, with negotiations currently ongoing.
The human body produces antibodies when it fights an infection, so by measuring antibodies in the blood doctors can detect whether someone has had the infection previously.
• Additional reporting by PA.