A British company who are supplying a crucial ingredient of the Covid-19 vaccine have warned that delays at the border after Brexit could prevent a “crucial step” in ensuring it is available to millions of people.
Croda International, based in Yorkshire, have provided a key chemical element of the vaccine to Pfizer in the trial phase, and has won a five-year contract that will see it deliver materials for 1.3 billion doses next year alone, worth around £75m.
But this comes just as the UK will introduce new custom controls from January 1, which could make distribution more challenging.
There have already been warnings that those wanting to cross the border could see delays of up to 48 hours.
MORE: Fears that Brexit could hold up coronavirus vaccine in the UK
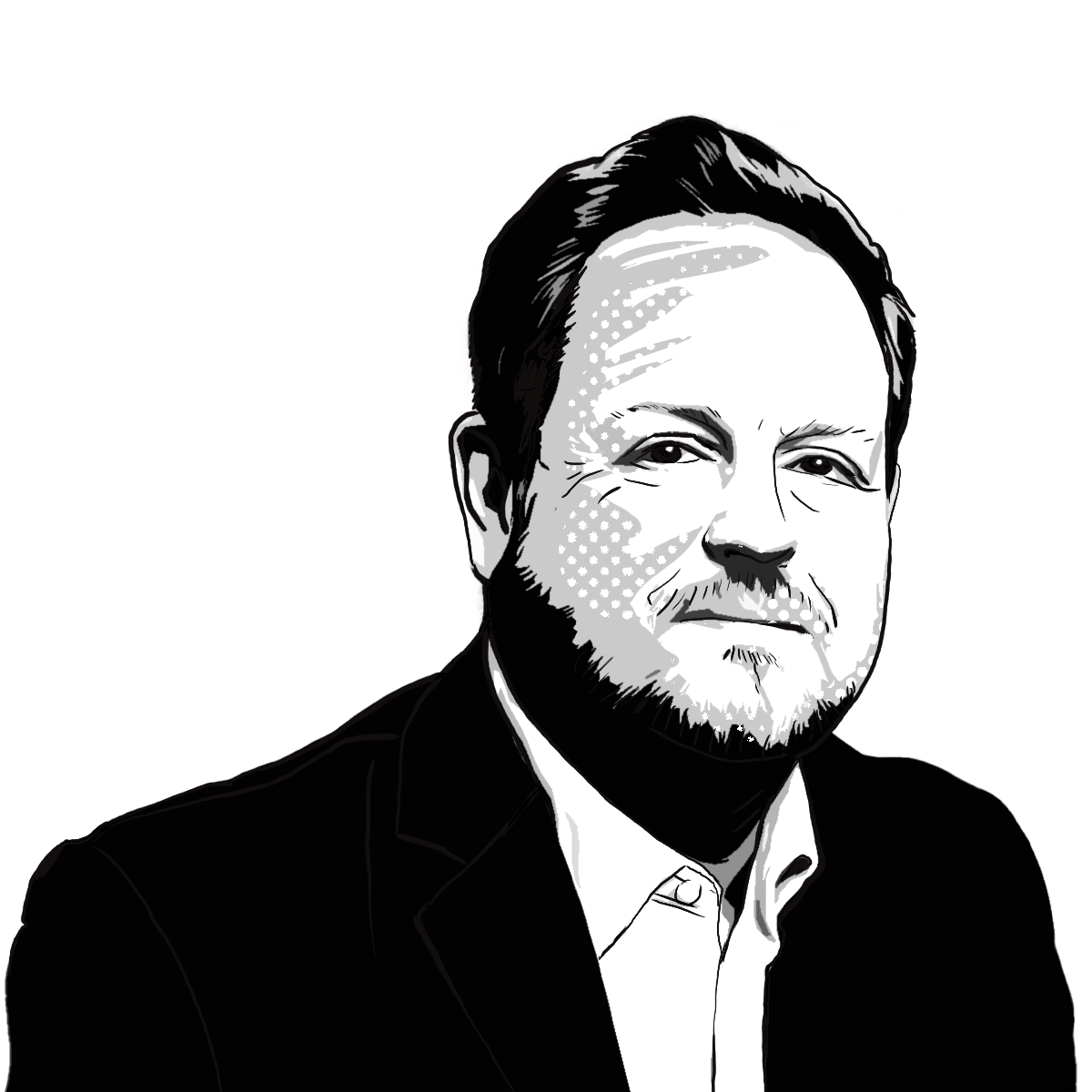
Croda’s chief executive Steve Foots told Sky News: “The worry of course, the last thing we need, is a problem with a lack of an agreement, and you’ve got friction at the borders, and I’m sure the UK government are acutely aware of this.
“We must make sure that the vaccine doesn’t have any problems getting into the UK, into the supply chain, or even the practical issues of refrigerant technologies and everything else.
“They could be products that are needed for the UK that are sourced abroad, so making sure that we are free from friction at the borders is is a crucial step for the vaccine.”
50 days before the Brexit transition period ends and with negotiations continuing on key areas to unlock a trade deal, the government said that it was launching a task force to help business prepare.
Following the success of the initial results, Mr Foots said that scaling up production from a 40,000-strong trial to produce and distribute 1.3 billion doses next year poses a major challenge.
“The public statements from Pfizer will tell you that they’re not there yet, there’s the safety clearance, which comes in November, hopefully, and then the big job through innovation is to scale up through the supply chain,” he said.
“We definitely have the quantities available for next year’s vaccine for Pfizer, they’re talking about 1.3 billion doses, and we’re ready to be able to supply that.
“But our mind is beyond next year and making sure we’ve got insurance in the supply chain to scale this up further, so we’re investing heavily in our units and factories running the UK, and one in the US.”